Keck School of Medicine of USC Alzheimer's Therapeutic Research Institute
2022 Annual Report

Chair's Message
Paul S. Aisen, MD Founding Director of Alzheimer's Therapeutic Research Institute, Professor of Neurology, Keck School of Medicine of USC
Paul S. Aisen, MD Founding Director of Alzheimer's Therapeutic Research Institute, Professor of Neurology, Keck School of Medicine of USC
2022 may be considered the culmination of three decades of work to develop disease-modifying treatments for AD. Establishment of trial methods, outcome measures, analytical approaches, biomarker standards, regulatory pathways; collaboration with industry; lessons from dozens of trials. See our blog posts on a few major publications:
* Treating Alzheimer's with Lecanemab: A Clinical Guide

* Early-Stage Alzheimer's disease: Getting Trial:Ready
* Open Letter: We Have Turned the Corner
By The Numbers
30%
slowing of early AD, cognitive decline possible
+30 years
of research for a disease-slowing therapy
+ 6 million
Americans are living with Alzheimer's
Collaborations

Alzheimer's Clinical Trial Consortium
The National Institutes of Health, National Institute of Aging grant, Alzheimer's Clinical Trial Consortium (ACTC) is a clinical trials infrastructure launched in December 2017 and is due for renewal every five years. The renewal application was submitted, reviewed, and received an Impact Score of 19. This is well within the fundable range. This score reflects the outstanding contributions and innovations of our network of Member Sites, Units, and Committees during the initial five years.
The original design, to accelerate and expand studies for therapies in Alzheimer's disease and related dementias will likely be renewed at the requested amount. ACTC's mission is to provide an optimal infrastructure, utilizing centralized resources and shared expertise, to accelerate the development of effective interventions for Alzheimer’s disease and related disorders.

Currently, we are coordinating many studies along the continuum of Alzheimer's disease (AD).
At the earliest stage, the first prevention trial to use innovative approaches to enrich samples with individuals who have elevated brain amyloid is the AHEAD 3-45 Study. We are testing if we can slow the accumulation of tau and prevent the cognitive decline associated with AD during its preclinical stage. The two sister trials (A3 and A45) in cognitively unimpaired (CU) individuals ages 55 to 80 are targeting both the preclinical and the early preclinical (intermediate amyloid) stages of AD and is the first secondary prevention trial to employ plasma-based biomarkers to accelerate the screening process and potentially reduce the number of screening PET scans.
On the other end of the continuum of AD we have received the notice of award and are planning to work with people diagnosed with Alzheimer's or other types of dementia who are receiving hospice care at the end of life in the LiBBY study.
"So our team at USC ATRI is focused on the development of effective treatments. We're not focused on one drug or one treatment or one stage of disease. We seek the most effective treatments of any kind. We share everything that we do. We're collaborative, and our data is shared widely."
One of the biggest challenges in the fight against Alzheimer's disease is that a therapeutic intervention must be initiated at the very early stages of the disease. Trial-ready cohorts are an effective strategy for the identification of participants eligible for these clinical trials, but building a cohort requires considerable planning.The recruitment of asymptomatic or minimally symptomatic study participants has sometimes taken 3+ years and more than 5,900 screens to recruit 1,169 participants. The challenges experienced by trial-ready cohorts have also included difficulties in recruiting an ethnically and racially representative group.
A new clinical trials infrastructure is required to increase the efficiency of recruitment and accelerate progress. Collaborations are now addressing this need by establishing trial-ready cohorts of individuals with preclinical and prodromal AD.
This study reviews the lessons learned from trial-ready cohorts that have been established, with the aim of informing future efforts toward efficient, cost-effective trial recruitment.

Alzheimer's Clinical Trial Consortium - Down Syndrome
The National Institutes of Health, National Institute of Aging grant, Alzheimer's Clinical Trial Consortium Down-Syndrome (ACTC-DS) is built on the clinical trials infrastructure. The main project, Trial-Ready Cohort – Down Syndrome (TRC-DS), is creating a group or ‘cohort’ of adults with Down syndrome who are interested in participating in clinical trials aimed at discovering treatments that will reduce the risk of developing Alzheimer’s dementia. TRC-DS has surpassed the original goal of 120 participants and now aims for 200. TRC-DS is an observational study being coordinated by the University of Southern California Alzheimer’s Therapeutic Research Institute (USC ATRI) and conducted across 20 international sites.
The planned Down syndrome clinical trial has been funded and researchers hope to enroll TRC-DS participants into the trial quickly.

Our team has established a collaboration with AC Immune and helping conduct the Phase I/II clinical trial of their anti-amyloid vaccine in people with Down syndrome. Michael Rafii, MD, PhD presented results at the Alzheimer's Association Internation Conference (AAIC). Rafii leads the work into how studying Down syndrome can help with Alzheimer's disease research.

ATRI Laboratory Information Management System
Effective management of biospecimens and data plays a critical role in Alzheimer's disease and related dementias (AD/ADRD) basic science, translational, and clinical research. Information systems that assist laboratory personnel in the proper handling, processing, analysis, storage, and sharing of samples and data are complex and costly. The ATRI Informatics section, led by Gustavo Jimenez-Maggiora, has developed a novel cloud-based Laboratory Information Management System (ATRI LIMS) to support the rapid growth of the ATRI Biomarker Laboratory and Biorepository, a state-of-the-art facility led by Robert Rissman.
ATRI LIMS is part of the ATRI Informatics Platform (ATRI-IP) hosted in a secure, HIPAA-eligible Amazon Web Services (AWS) cloud computing environment. It supports centralized, scalable, auditable specimen management configured to meet study-specific requirements, thus ensuring that specimen handling, processing, and analysis are standardized, minimizing waste and duplication, and preserving the specimen chain of custody. ATRI LIMS supports integration and interoperation with other clinical trial and research information management systems, such as the ATRI EDC or third-party IT systems, via secure application programming interfaces (APIs). This capability enables the implementation of complex, multi-system data and specimen flows to support research conduct. Finally, the ATRI LIMS capabilities provide the underpinnings for a broader set of lab modernization initiatives focused on improving the efficiency and quality of ATRI laboratory, biobanking, and data- and sample-sharing methods that include the expanded use of artificial intelligence (AI) and sensor-based technologies (IoT, RFID), networked analysis instruments, robotics, and advanced reporting and visualization, and predictive analytics tools. Once implemented, these initiatives will accelerate scientific discovery, streamline lab operations, and improve data quality while reducing costs. Recent research in Alzheimer's disease and related dementias (AD/ADRD) has shown promising results, with several drugs demonstrating potential in clinical trials. Conducting multicenter clinical trials for AD/ADRD is a complex process that requires the collection and management of vast amounts of data from multiple sources. To ensure the success of these trials, ATRI's Informatics Section, led by Gustavo Jimenez-Maggiora, has developed the ATRI electronic data capture system (ATRI EDC).

ATRI Data Sharing Initiative
Since its founding, ATRI has been committed to the principles of Open Science and data sharing2,3 by ensuring broad access to data, software, instruments, biospecimens, and expertise in trial design. To achieve this goal, the ATRI Informatics section, led by Gustavo Jimenez-Maggiora, is developing a new research IT platform: ATRI DataShare. This platform supports:
· Visualization and linkage of materials to enable precise requests
· Selection of data results (biomarker, clinical, and imaging), images, and biospecimens by demographic, genetic, and other data specifications
· Sharing of images that are de-identified and defaced (where necessary)
· Sharing of de-identified and/or anonymized biosamples
· Packaging of data sets with source code to facilitate analysis and reproducibility
· Sharing of extensive documentation including publications, protocols, consent forms, and data dictionaries
A recent example illustrates the impact of ATRI's data sharing efforts. In 2019, a rich, first-of-its-kind dataset that includes clinical, neuropsychological, biomarker, and neuroimaging data from the A4 study was made available to the AD scientific community. As of February 2023, this dataset has been accessed by more than 1,200 investigators from academic, private, and public institutions in more than 60 countries (Figures 1, 2, 3). These data have informed the design of new studies and provided supporting evidence for new grant funding and publications. The ATRI EDC system uses agile, cloud-native software development methods to ensure that it can adapt to rapidly evolving research designs and technologies. The system is customizable to meet the specific needs of individual studies and supports a high degree of study-specific configurations. It also encourages collaboration among researchers and promotes the principles of open science.
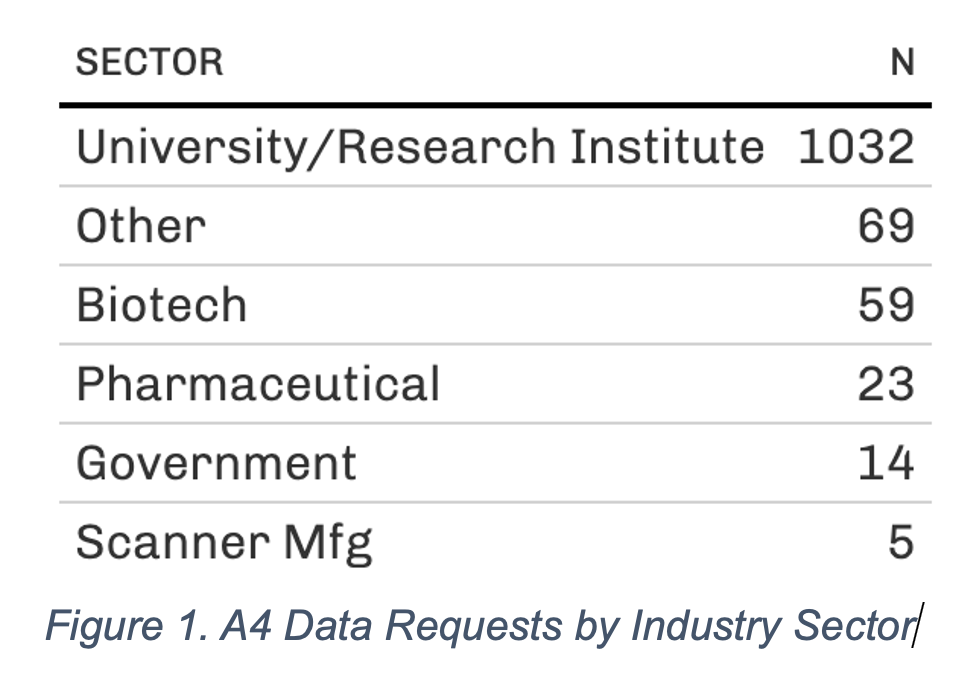
Figure 1
Figure 1
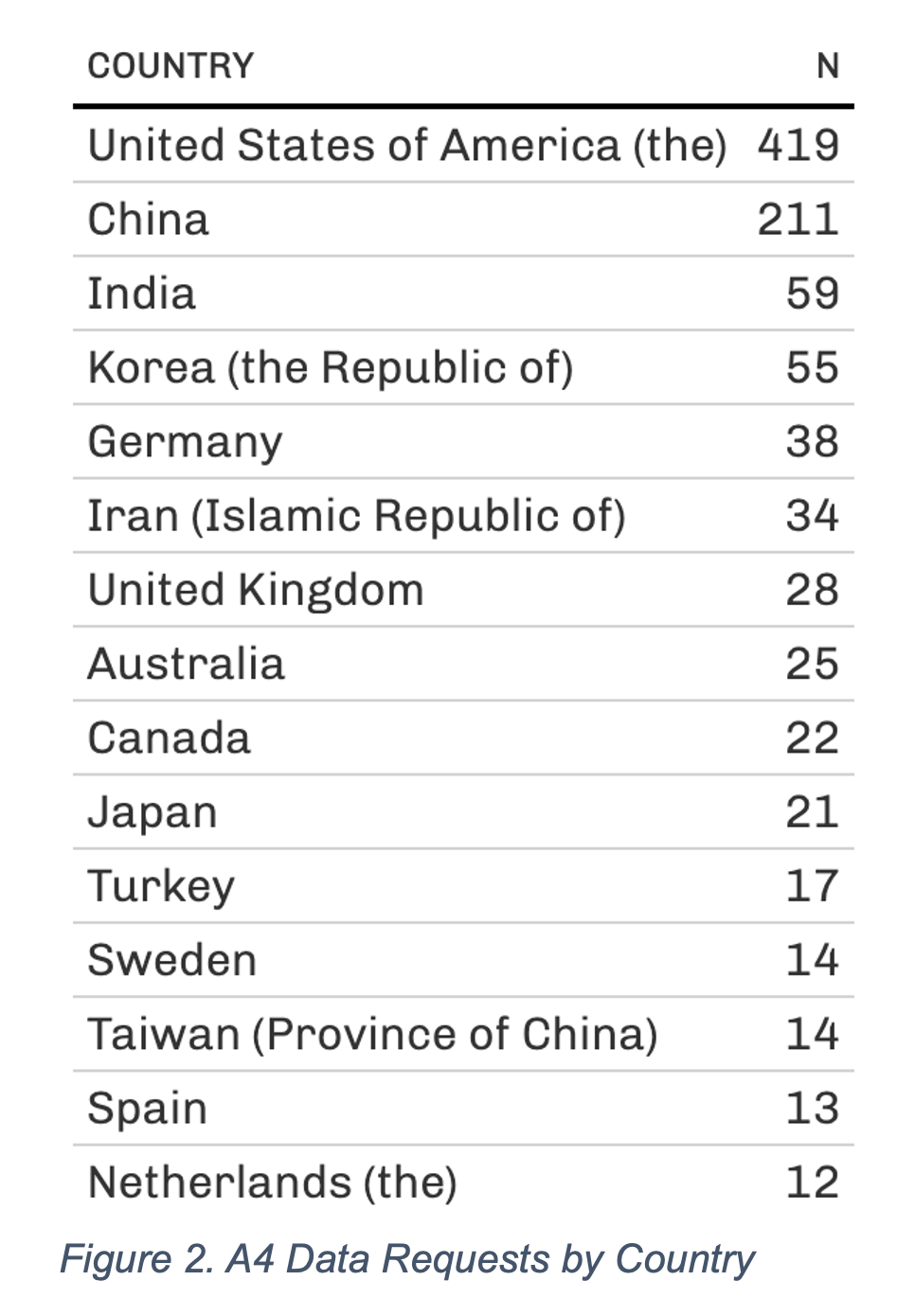
Figure 2
Figure 2
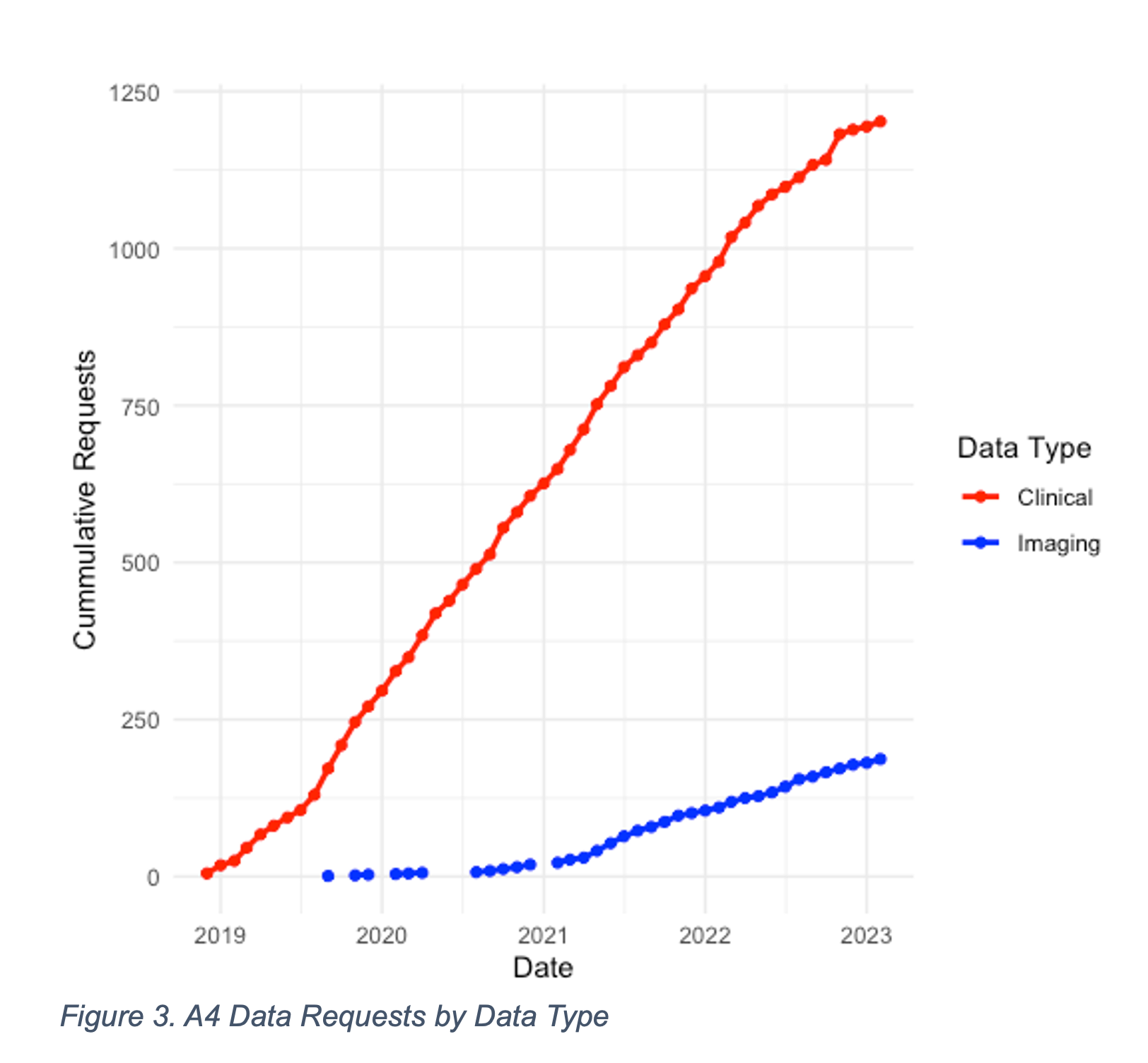
Figure 3
Figure 3
Rapidly Growing Number of Clinical Trials and Observational Studies Under Management
ATRI's large portfolio of more than 20 AD multi-site clinical trials and observational studies continued its rapid growth in 2022-2023, with six new studies, including two "gold-standard" RCTs, scheduled to launch in the first half of 2023. These studies aim to collect and explore novel outcomes (fluid and imaging biomarkers, cognitive, pharmacological, safety) in multiple AD populations - late-onset, early-onset, and familial - resulting in complex designs that require the efforts of multidisciplinary study teams for successful implementation. The ATRI Informatics section, led by Gustavo Jimenez-Maggiora, provides clinical data management (CDM) leadership and oversight for ATRI studies, working closely within each multidisciplinary study team to develop study databases that are accurate, secure, reliable, and analysis-ready in an effective manner.

The centerpiece of CDM's study management approach is the development of a data management plan (DMP), a multistakeholder document that provides a blueprint for a study's data management processes: Roles and responsibilities, decision making, data collection, data handling, data quality control, and reporting. The DMP governs data management activities during the entire study lifecycle, from initiation to closeout. Key inputs to the DMP include the study protocol, statistical analysis plan (SAP), data flows, and scope of work (SOW)4. The DMP methodology guides the CDM team's development and management of each study database providing a stable infrastructure that allows ATRI to continue to support an increasing number of studies, accelerate new scientific advances, and increase its impact.
Studies launching in 2022-2023:
· Alzheimer's Disease Neuroimaging Initiative - ADNI4
· Synaptic Therapy Alzheimer's Research Trial - START
· Life's End Benefits of Cannabidiol and Tetrahydrocannabinol - LiBBY
· AHEAD Plasma Extension Study - APEX
· Biomarker Evaluation in Young Onset Dementia from Diverse Populations - BEYONDD
· Alzheimer'd Clinical Trials Consortium ACTC Brain Donation Registry

Education & Training

IMPACT-AD
The Institute on Methods and Protocols for Advancement of Clinical Trials is an Alzheimer’s Disease and related disorders (IMPACT-AD) course that aims to educate and promote diversity among research professionals and future principal investigators in the field of ADRD research.
The primary aim is to expose students to the science of conducting clinical trials for AD
This year the course was held in person and provided early-stage investigators and those new to Alzheimer's trials with a unique, comprehensive, and active learning experience in Alzheimer's disease and related dementias (ADRD) trials.


IMPACT-AD at AAIC in San Diego
IMPACT-AD at AAIC in San Diego
Alzheimer's Trial Recruitment Innovation Lab Fellowship Program
A two-year program is designed to train and develop the next generation of researchers interested in Alzheimer’s disease and related dementias (ADRD) clinical trials through a multidisciplinary, active, team-based learning approach.
The fellowship combines rigorous didactic sessions, clinical trial methods training, hands-on experience working on ATRIL’s research projects, and a mentored clinical research project using previously collected data. The training program is enhanced through interactive seminars, workshops, and other learning and networking opportunities.
The fellowship is funded through a grant by the American Heart Association’s Strategically-Focused Research Network (SFRN) on the Science of Diversity in Clinical Trials along with the USC Schaeffer Center for Health Policy and Economics, the USC Alzheimer’s Therapeutic Research Institute (ATRI) and Howard University. The fellowship stipend is $85K per year.

Rema Raman, PhD Director, Alzheimer’s Trial Recruitment Innovation Lab, Professor of Neurology, USC Keck School of Medicine Director, Section of Biostatistics & Section of Participant Recruitment & Retention, USC Alzheimer’s Therapeutic Research Institute
Rema Raman, PhD Director, Alzheimer’s Trial Recruitment Innovation Lab, Professor of Neurology, USC Keck School of Medicine Director, Section of Biostatistics & Section of Participant Recruitment & Retention, USC Alzheimer’s Therapeutic Research Institute

Jevay Grooms, PhD Co-Director, Alzheimer’s Trial Recruitment Innovation Lab Co-Director, Center for Equitable Economy and Sustainable Society (E2S2) Assistant Professor, Economics Department, Howard University Visiting Distinguished Scholar, USC Price School of Public Policy
Jevay Grooms, PhD Co-Director, Alzheimer’s Trial Recruitment Innovation Lab Co-Director, Center for Equitable Economy and Sustainable Society (E2S2) Assistant Professor, Economics Department, Howard University Visiting Distinguished Scholar, USC Price School of Public Policy

Mireille Jacobson, PhD Principal Investigator, Alzheimer’s Trial Recruitment Innovation Lab, Associate Professor and Assistant Dean of Faculty and Academic Affairs, USC School of Gerontology Co-Director, Aging and Cognition Program, USC Schaeffer Center
Mireille Jacobson, PhD Principal Investigator, Alzheimer’s Trial Recruitment Innovation Lab, Associate Professor and Assistant Dean of Faculty and Academic Affairs, USC School of Gerontology Co-Director, Aging and Cognition Program, USC Schaeffer Center

Cecily Jenkins, PhD Principal Investigator, Alzheimer’s Trial Recruitment Innovation Lab, Associate Professor of Clinical Neurology, USC Keck School of Medicine Director of Neuropsychology, USC Alzheimer’s Therapeutic Research Institute
Cecily Jenkins, PhD Principal Investigator, Alzheimer’s Trial Recruitment Innovation Lab, Associate Professor of Clinical Neurology, USC Keck School of Medicine Director of Neuropsychology, USC Alzheimer’s Therapeutic Research Institute

Doris P. Molina-Henry, PhD Principal Investigator, Alzheimer’s Trial Recruitment Innovation Lab, Assistant Professor of Research Neurology, USC Keck School of Medicine Alzheimer’s Therapeutic Research Institute
Doris P. Molina-Henry, PhD Principal Investigator, Alzheimer’s Trial Recruitment Innovation Lab, Assistant Professor of Research Neurology, USC Keck School of Medicine Alzheimer’s Therapeutic Research Institute
Summer Student Research Internship
The USC Keck School of Medicine Alzheimer’s Therapeutic Research Institute (ATRI) in San Diego, CA, offered an in-person Summer Student Research Internship Program for undergraduate, graduate, and professional students. All interns work closely with and learn from an internationally renowned, multi-disciplinary team of scientists, researchers, and staff members in Alzheimer’s Disease (AD) clinical trials research.
The primary aim is to expose students to the science of conducting clinical trials for AD
The internship program exposes students to the science of conducting clinical trials for AD and provides a unique opportunity to explore careers in AD therapeutic research while developing research skills and professional experience. In addition to the completion of a focused summer project, students will develop connections with other ATRI interns and staff and will be integrated into the ATRI network and community.
Interns will learn about their day-to-day tasks through their specific work assignments and interact with Institute Directors, faculty, and their teams through a series of seminars and professional development workshops. Interns will be required to submit an end-of-project report, approved by their ATRI mentor. The report will summarize the research/creative project, intern activities, and culminates in a presentation at the Annual ATRI Summer Student Research Seminar

Mentorship
Michael Rafii, MD, PhD serves as faculty mentor for NIH K23 awardee, Dr. Jonathan Santoro, a pediatric neurologist at Keck School of Medicine and Children’s Hospital of Los Angeles who studies the immune system in persons with Down syndrome.
The K23 program is designed to provide "protected time" for clinically trained individuals to receive intensive, supervised research training in biomedical research related to neurological disorder
Together, they have been conducting studies to better understand the role of the immune system in neurological diseases such as cerebrovascular disease and Alzheimer’s disease in this population. Their collaboration has resulted in numerous publications and represents a strong interdisciplinary collaboration focused on Down syndrome across the lifespan.

ATRI Expands Scope

Lab & Biorepository
In cooperation with Carolyn Meltzer, Dean of the Keck School of Medicine and Berislav Zlokovic, Director, Zilkha Neurogenetic Institute we successfully recruited our long-term collaborator Robert Rissman, Director of ATRI Biomarker Laboratory and Biorepository to establish a Neuroscience Translational Research Division. This change greatly expands the scope of ATRI to include basic science translational research in models of disease that can serve as preclinical work for future therapeutics. Rissman is appointed to the Department of Physiology and Neuroscience as a tenured professor. Rissman has also been appointed to the W.M. Keck Endowed Chair in Medicine. Robert's team now occupies a 45,000-square-foot building adjacent to our existing building in the Sorrento Mesa section of San Diego. During the coming year, we will complete the build-out of the laboratory facilities in the building allowing us to accommodate additional basic and clinical research faculty. A major focus will be the continued development of plasma biomarkers of AD neurobiology.
So far we have expanded our biorepository within the new space:




Initiatives, Top Stories & News
Innovation
The Alzheimer's disease continuum begins with a long asymptomatic or pre-clinical stage. We launched the first secondary prevention trial to employ blood biomarkers to accelerate the screening process and potentially substantially reduce the number of PET scans.

Impact
Making studies more inclusive and representative is imperative. Rema Raman, Ph.D. established the IDEA-CT, Inclusion, Diversity, and Education in AD/ADRD Clinical Trials, unit, guided by the principles of justice, equity, diversity, and inclusion (JEDI).

Funding
The creation of the Epstein Family Alzheimer's Research Collaboration is supporting new collaborative efforts to discover effective therapies for Alzheimer's disease.

Participants Rights
ATRI's Research Participant Advisory Board recently met and celebrated the publication of a new paper titled "Disclosing Individual Results in Dementia Research: A Proposed Study Participant’s Bill of Rights." The "Bill of Rights" is a call to action for researchers in Alzheimer’s disease and related dementias (ADRD) to proactively design clinical studies that provide the option for research participants to learn their individual research results if they choose, and in a manner that ensures study integrity.

New Methods
Launch of AlzMatch, a new research study where selected volunteers have their blood tested to determine potential eligibility for Alzheimer's disease trials.
The AlzMatch Study seeks to determine whether a single blood test, collected at local laboratories across the country, can help speed clinical trial enrollment for anti-amyloid treatments by identifying individuals who have an increased likelihood of having brain amyloid.

Revolutionizing Alzheimer's Clinical Trials
Recent research in Alzheimer's disease and related dementias (AD/ADRD) has shown promising results, with several drugs demonstrating potential in clinical trials. Conducting multicenter clinical trials for AD/ADRD is a complex process that requires the collection and management of vast amounts of data from multiple sources. To ensure the success of these trials, ATRI's Informatics Section, led by Gustavo Jimenez-Maggiora, has developed the ATRI electronic data capture system (ATRI EDC).
Faculty

Faculty & Section Director Profiles

Michael Rafii, MD, PhD
Professor of Neurology, Medical Director of Alzheimer's Therapeutic Research
Research Areas: • Clinical Trials for Alzheimer's disease in Down syndrome
• Biomarkers of Alzheimer's disease in Down syndrome
• AD Clinical Trial Design and Conduct

Rema Raman, PhD
Professor of Neurology; Director, Section of Biostatistics & Section of Participant Recruitment & Retention Alzheimer's Therapeutic Research Institute
Research Areas:
• Biostatistics
• Clinical trial design, conduct and analysis
• Participant recruitment and retention
science

Robert Rissman, PhD
Director of the Neuroscience Translational Research Division and ATRI Biomarker Laboratory and Biorepository, Professor of Physiology and Neuroscience and Endowed Professor in Medicine, USC
Research Areas: • Preclinical studies of Alzheimer’s Disease and Related Dementias (ADRD) • Experimental neuropathology of ADRD • Novel biomarker discovery

Cecily Jenkins, PhD
Associate Professor of Clinical Neurology; Director of Neuropsychology, USC Alzheimer’s Therapeutic Research Institute
Research Areas:
• AD Clinical trial design
• Remote assessment of cognitive risk for future AD
• Innovative measurement of cognition in pre-clinical AD

Michael Donohue, PhD
Professor of Neurology, Associate Director of Biostatistics, Alzheimer's Therapeutic Research Institute
Research Areas
• Alzheimer's clinical trial design and
analysis
• Statistical modeling and prediction of
Alzheimer's disease progression
Gustavo Jimenez-Maggiora, MBA, PhD Candidate
Director of Informatics Section
Research Areas:
• Clinical Research Informatics
• Remote Data Capture Systems
• Natural Language Processing

Volunteer

Connect With Us
© 2023 Keck School of Medicine of USC. All rights reserved.



